Navigating the European Union Artificial Intelligence Act for Health – npj Digital Medicine
The European Union (EU) has taken a serious step to regulate artificial intelligence (AI) with the adoption of the AI Law by its 27 member states on March 13, 2024.1. The first was presented in April 2021 by the European Commission2The AI Act arose out of a growing recognition of the transformative power of AI and the need to address ethical risks, building on previous EU initiatives, including the 2018 Coordinated Plan on AI.3 and the 2020 White Paper on AI4. The Council issued its position in December 20225followed by the European Parliament’s adoption of its negotiating position in June 20236. After updating the plan, the final negotiations between the Commission and the Council resulted in an interim agreement in December 2023, which was signed by the Members in February 2024.1.
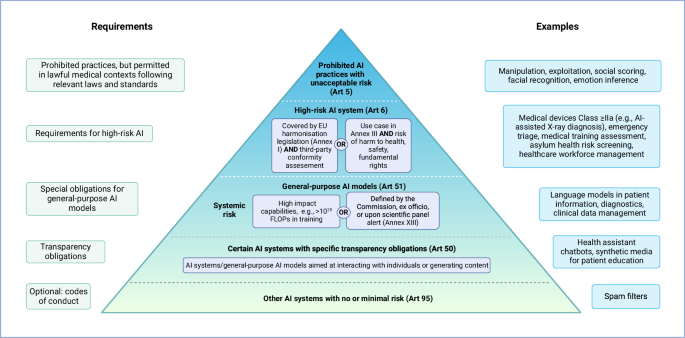
As the world’s first comprehensive legislative framework specifically on AI, the AI Act aims to promote human-centered and trustworthy AI while protecting health, safety and fundamental rights of humans from the potentially harmful effects of AI-enabled systems (Article (Art) 1 (1)). The law provides harmonized rules for placing on the market, implementation and use of AI systems and has become binding law in all EU Member States 20 days after its publication in the Official Journal of the EU (published on July 12).th 2024), regardless of existing national laws and guidelines on AI (Art 1 (2a), Art 113). Most of the regulatory elements will come into force within 24 months, with restrictions, that is, restrictions on AI applications that are considered to cause unacceptable risk, which will come into force within 6 months (Art 113 ( (a–c)).
In its current form, the AI Law has a far-reaching scope, not only in the internal market but also abroad, as it applies to all providers of AI applications in the EU market , regardless of where they are established or located (Art 2). (1a) see definitions of provider, supplier, and AI system in Table 1). In addition, the AI Law applies to providers and operators of AI systems in third countries if the resulting product is used in the Union (Art 2 (1c)). However, ‘output’ is not defined in the AI Act, and the examples are vague, such as “[…] predictions, content, recommendations, or decisions that may affect the physical or virtual environment […]” (Art 3 (1)). This suggests that any AI product can comply with the AI Law if its production is accepted in the Union, regardless of the purpose or location of the supplier or agent or location. Finally, the AI Act applies to “[…] aliens of AI systems having their place of establishment or located within the Union […]” (Art 2 (1b)). Therefore, operators of AI systems within the EU, even if their models are not intended for the EU market, must comply with the rules of the AI Act.
For the health care area, the AI Act is particularly important, as are other existing consensus rules, such as the Medical Device Regulations (MDR; it regulates products with an intended medical purpose In the EU market, it distinguishes them from the first low-risk group (eg bandages) to the high-risk Group III (eg implanted pacemaker)) or the In Vitro Diagnostic Medical Device Regulation (IVDR; regulates in vitro diagnostic equipment for medical purposes intended for the EU market, classifying them from Group A (low risks, e.g. laboratory equipment) to Group D (high risk growth, for example, products to detect highly contagious viruses such as Ebola)), do not clearly cover medical AI applications. Furthermore, not all AI applications that can be adopted in the healthcare sector fall within the MDR or IVDR, for example, many large language models (LLM) such as ChatGPT.2,3.
Due to the large impact of this law on the market, all those involved in the field of medical AI, including manufacturers, providers, patients and doctors, can benefit from understanding the complex definitions, obligations and their needs. Understanding the framework will help to clarify which examples are covered by this law and the responsibilities that must be followed, to ensure that AI is implemented safely and responsibly, to prevent potential harm become available, and ultimately drive new trends in medical AI. In this explanation, we run through the most important points of the AI Act with the aim of the healthcare sector and provide easy references to follow the relevant chapters.
#Navigating #European #Union #Artificial #Intelligence #Act #Health #npj #Digital #Medicine